
No accumulation was evident after repeated once daily administration of IFNγ-1b 100 μg/m 2 subcutaneously for 12 days. Mean elimination half-lives were 0.6, 2.9 and 5.9 hours after intravenous, intramuscular and subcutaneous injection, respectively, while mean peak plasma concentration occurred at 4 hours (1.5 μg/L) and 7 hours (0.6 μg/L) after intramuscular and subcutaneous injection, respectively. The greatest therapeutic benefit was found in patients aged 89% of the dose absorbed). The relative risk of serious infection and the number of days in hospital were each reduced by about two-thirds, and the mean duration of hospital stay by about one-third in those who did experience infection. Long term treatment with a therapeutic dosage of IFNγ-1b produced a significant reduction in the incidence of serious clinical events necessitating hospitalisation.
#COPAY ASSISTANCE INTERFERON GAMMA 1B TRIAL#
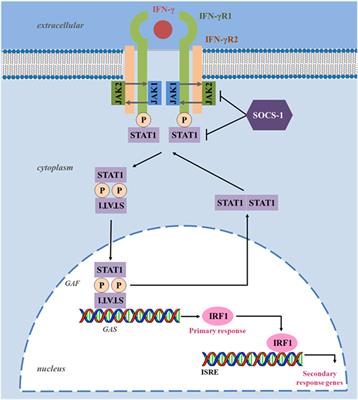
Following the finding that interferon gamma-1b (IFNγ-1b) can potentiate phagocyte activity in some other disease states as well as restoring defective phagocyte NADPH oxidase system activity in at least some patients with chronic granulomatous disease, a large-scale placebo-controlled trial was undertaken with IFNγ-1b in patients with chronic granulomatous disease. It is manifested by a common phenotype consisting of recurrent serious, life-threatening infection and granuloma formation. The FDA-approved product labeling can be found at or 1-86.Chronic granulomatous disease is a group of rare x-linked or autosomal genetic disorders of the phagocytic NADPH oxidase system involved in host defence against various microorganisms. To learn more, talk about ACTIMMUNE with your healthcare provider or pharmacist. The risk information provided here is not comprehensive. You are encouraged to report negative side effects of prescription drugs to the FDA. Tell your doctor about all other medications you are taking.Īvoid taking ACTIMMUNE at the same time as a vaccination. Some drugs may interact with ACTIMMUNE to potentially increase the risk of damage to your heart or nervous system, such as certain chemotherapy drugs.
What other medications might interact with ACTIMMUNE? Acetaminophen may be helpful in preventing fever and headache. Bedtime administration of ACTIMMUNE may help reduce some of these symptoms. The most common side effects with ACTIMMUNE are “flu-like” symptoms such as fever, headache, chills, muscle pain, or fatigue, which may decrease in severity as treatment continues. Your doctor will monitor these cells with blood tests at the beginning of therapy and at 3-month intervals on ACTIMMUNE therapy have, or have had, reduced bone marrow function.
have a history of seizures or other neurologic disorders.have a cardiac condition such as irregular heartbeat, heart failure, or decreased blood flow to your heart.are pregnant or plan to become pregnant or plan to nurse.What should I tell my healthcare provider?īe sure to tell your doctor about all the medications you are taking. If you experience a serious reaction to ACTIMMUNE, discontinue it immediately and contact your doctor or seek medical help. In rare cases, ACTIMMUNE can cause severe allergic reactions and/or rash. Your doctor should monitor your liver function every 3 months, and monthly in children under 1 year. Taking ACTIMMUNE may cause reversible changes to your liver function, particularly in patients less than 1 year old. This effect, which can be severe, is usually reversible when the drug is discontinued or the dose is reduced. These symptoms are usually reversible within a few days upon dose reduction or discontinuation of therapy.īone marrow function may be suppressed with ACTIMMUNE, and decreased production of cells important to the body may occur. What warnings should I know about ACTIMMUNE?Īt high doses, ACTIMMUNE can cause (flu-like) symptoms, which may worsen some pre-existing heart conditions.ĪCTIMMUNE may cause decreased mental status, walking disturbances, and dizziness, particularly at very high doses. When should I not take ACTIMMUNE?ĭon’t use ACTIMMUNE if you are allergic to interferon-gamma, E coli-derived products, or any ingredients contained in the product.


SMO is a genetic disorder that affects normal bone formation and is usually diagnosed in the first few months after birth. ACTIMMUNE is also used to slow the worsening of severe, malignant osteopetrosis (SMO).


 0 kommentar(er)
0 kommentar(er)
